Instructions by Specimen Type
Microbiology Organisms
Step 1:
Verify That the Specimen is a Microbiological Actively Growing, Pure Culture
In the Test Catalog, use the Specimen tab and the Clinical and Interpretive tab of each test to identify:
- Specimen requirements
- Specimen container requirements
- Specimen stability (temperature) requirements
- Required forms or special instructions
Containers must be capable of withstanding, without leakage, an internal pressure of 95 kPa in the range of -40C to 55C.
Note: Mayo Clinic Laboratories does not accept culture plates. You must subculture organisms onto a slant prior to submitting the specimen for testing.
Step 2:
Label the Specimen
Specimens must have two person-specific identifiers on the patient label. Person-specific identifiers include:
- Accession number
- Patient's first and last name
- Unique identifying number (for example, medical record number)
- Date of birth
Mislabeled Specimens
Specimens are considered mislabeled when there is a mismatch between the person-specific identifiers on the specimen and the information accompanying the specimen. This information might include a computer system, requisition form, or additional paperwork.
In addition, if a handwritten name and a label are on the container, the information must match exactly. For example, "Rebecca" does not match "Becky." When insufficient or inconsistent identification is submitted, a new specimen may be required.
Step 3:
Place the Culture in a Secondary Leak-Proof Container
Containers must be capable of withstanding, without leakage, an internal pressure of 95 kPa in the range of -40°C to 55°C.

T146 Infectious Container
The Infectious Container (T146) is leakproof and includes bubble wrap (to prevent breakage) and an absorbent material inside. If multiple microbiology specimens are sent, each specimen must be transported in its own container.
Step 4:
Package the Specimen in a Biohazard Bag
Place the tube or container in a Mayo Clinic Laboratories color-coded (temperature-specific) shipping bag.

T043 Biohazard Bag - 12x15 Use if your container is too large for color-coded bags. Mark it Frozen, Refrigerate, or Room Temp (Ambient).
Electronic Clients:
- Clients who submit electronic orders will have a batch order. Place all specimens for the temperature-specific batch number into one bag. If all the specimens do not fit, use a larger biohazard bag (T043) and indicate the shipping temperature.
- Do not place multiple batches into one bag.
- The bag must be leakproof.
- There must be absorbent material between the primary receptacle (tube/container) and the secondary packaging (bag) that is able to absorb the entire contents of the bag.
- Wrap any breakable tubes individually in bubble wrap (T055).
Step 5:
Package the Specimen

Folded batch sheet with bar code and delivery address visible
Electronic Clients
Clients who submit electronic orders will have a batch sheet. The bottom of the batch sheet lists the number of pages (for example, 1 of 3). Fold the batch sheets into fourths and place them in the outside pocket of the bag. If there is no pocket, place them inside the bag with the specimens. Include all pages in the corresponding bag. The delivery address and bar code, if applicable, must be visible. Do not combine multiple batches into one bag.
Manual Clients
Clients who do not order electronically must include a completed Test Request form with each patient specimen. Fold and insert the completed form into the outside pocket of the biohazard bag. If there is no pocket, place the form inside the bag with the specimen.
Step 6:
Place a Blue "C" Culture Label on the Bag

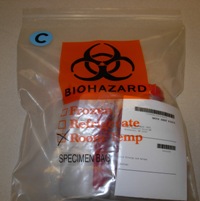
Biohazard bag with two containers, folded batch sheet with bar code visible, and C sticker.
Verify that your test appears on the Microbiology Culture Test List. Place a blue "C" culture label (T549) on the shipping bag to identify it as an actively growing culture.
It is also acceptable to place the C sticker on the batch sheet, making sure the C sticker shows through the bag when the batch sheet is folded.




